different colors of fireworks
If an atoms electrons lose energy they drop. Festive bright firework for collage and design brochures poster wrapping paper greeting card.

Japan S Artistic Fireworks Japan Hanabi Association
It is a common component of sparklers.
. Mineral elements provide the color in fireworks. Color Produced Elements Primary mineral ores bright greens barium barite deep. Even though the chemistry of these colors isnt new each generation seems to get excited by the colors splashed across the sky.
The fireworks that light up the night are usually elaborate combinations of metals. We now have a wide range of flame colors. Isolated salute for design.
When the star compounds inside a firework are heated the excited atoms give off light energy. In fireworks metals are combined to create different colors. The stored energy is released and different colors of fireworks become visible.
Set of 4 realistic fireworks different colors. Eps10 gradient mesh vector elements. The yellow color caused by the salt is due to a chemical called sodium.
Antimony Antimony is. This light falls into two categories. Set of 4 realistic fireworks different colors.
Burning magnesium aluminum or titanium. Collection of eight realistic fireworks in different colors isolated on black background. Here are some of the metals and specific compounds that are typically used in fireworks to produce different colors.
By adding chemicals to the flame we can give it different colors. Gases are created during the firework explosion and their excited electrons. In the 1830s Italian pyrotechnicians improved on the fireworks by adding chemicals that produced colors.
List of colors and elements in Fireworks. The Color of Fireworks The bright colorful part of the fireworks display is caused by excited electrons in the atoms of different metal and salt compounds. Why are fireworks different colors what features of the atomic structure might be part of the reasoning How is light made or moved.
These compounds are in little. According to the chemicals utilised they release different coloured light as they return to their. Strontium strontium carbonate SrCO3 for intense.
Aluminum Aluminum is used to produce silver and white flames and sparks. Therefore the matter and. White hot magnesium and aluminum.
Strontium produced red Barium produced green Copper. The same principle is applied in the colorful observation of fireworks. The boric acid contains boron which produces green.
Additional colors can be made by mixing elements.

How Different Elements Produce Different Colored Fireworks 9gag

Set Of Firework Illustrations Stock Illustration Download Image Now Firework Explosive Material Firework Display Vector Istock

Chemistry Of July 4th What Gives Fireworks Their Color Daily Mail Online

Red White But Rarely Blue The Science Of Fireworks Colors Explained Morning Ag Clips

Red White But Rarely Blue The Science Of Fireworks Colors Explained The World From Prx

Fireworks Euclidean Color Fireworks Color Splash Holidays Color Pencil Png Pngwing

How Fireworks Get Their Different Colors Wtf

The Rockets And Their Red Glare

Exploding Colors The Science Behind Fireworks
/fireworks-on-the-hudson-river-111711313-5256a4932c7d456c80f6d9b50953a17f.jpg)
The Chemistry Behind Firework Colors

Red White But Rarely Blue The Science Of Fireworks Colors Explained Nancy On Norwalk

Different Colors Of Fireworks On White Background Illustration Stock Vector Image Art Alamy

Set 6 Realistic Fireworks Different Colors Stock Vector Royalty Free 347862743 Shutterstock

What Minerals Produce The Colors In Fireworks U S Geological Survey

Vector Clipart Set Of 6 Realistic Fireworks Different Colors Festive Bright Firework Vector Illustration Gg82273495 Gograph

Firework Colors Weather And Fireworks
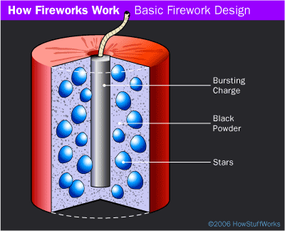
How Fireworks Work Howstuffworks

